|
Research projects
Electrospinning and characterization of
supramolecular polymer complexes
Electrospinning
is a simple and flexible method that uses a large electric field to produce
polymeric fibers with diameters ranging from a few mm to 50 nm, much
below that of conventional methods. These fibers generate a growing interest
for applications in tissue engineering, smart membranes, nanocomposites,
etc. However, the properties of these fibers are often poorly controlled,
limiting their potential use. Metastable or unusual structures are sometimes
produced because of the rapid solvent evaporation, extensional forces, and
intense electric field involved in electrospinning. Contrary to expectations,
electrospun fibers often present a low level of molecular orientation that
can lead to deceiving properties.
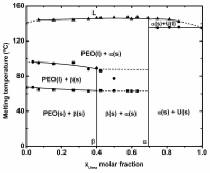 We have recently
shown for the first time that nanofibers of supramolecular complexes between poly(ethylene oxide) (PEO) and urea (U) can be prepared by
electrospinning. The fibers show exceptionally large orientation levels, the
largest (to our knowledge) ever published for electrospun polymers. We also
used electrospinning to prepare for the first time pure samples of the b PEO-U complex.
We have determined its structure and have demonstrated using time-resolved IR
spectroscopy that it is in thermodynamically stable, in spite of numerous
reports of metastability. We are currently studying
the electrospinning of complexes between various polymers/small molecules
system to fully explore the benefits of these complexes. We also use
electrospinning as a preparation method to allow a better characterization of
supramolecular complexes. We have recently
shown for the first time that nanofibers of supramolecular complexes between poly(ethylene oxide) (PEO) and urea (U) can be prepared by
electrospinning. The fibers show exceptionally large orientation levels, the
largest (to our knowledge) ever published for electrospun polymers. We also
used electrospinning to prepare for the first time pure samples of the b PEO-U complex.
We have determined its structure and have demonstrated using time-resolved IR
spectroscopy that it is in thermodynamically stable, in spite of numerous
reports of metastability. We are currently studying
the electrospinning of complexes between various polymers/small molecules
system to fully explore the benefits of these complexes. We also use
electrospinning as a preparation method to allow a better characterization of
supramolecular complexes.
Development and
application of PM-IRSAS
Infrared linear
dichroism (IRLD) is a powerful method to study orientation and structure in
deformed polymers. Its main strength is its selectivity that allows probing
simultaneously the different phases of semi-crystalline materials, the
components in blends, the blocks in copolymers, or specific moieties in
complex systems. Its
main limitation is its low time resolution. Polarization modulation IRLD
solves part of this problem by providing a 200 ms
time resolution and a much improved sensitivity, but at the expense of the
loss of the individual polarized spectra. This limits the structural
information available and can lead to quantification errors if
crystallization or conformational changes occur. We have very recently
demonstrated for the first time a novel method, polarization modulation
infrared structural absorbance spectroscopy (PM-IRSAS), that allows recording
the dichroic difference spectrum and the individual p- and s- polarized
spectra (and from these the structural absorbance spectrum) in a single 200 ms scan. PM-IRSAS therefore combines the speed and sensitivity
of PM-IRLD with the rich information of standard IRLD. We demonstrated its
performance by studying the deformation of poly(ethylene
terephthalate) (PET) thin films as well as its cold crystallization. The
importance of this work was recognized by the SAS Meggers
Award for the best paper published in Applied Spectroscopy in 2008. systems. Its
main limitation is its low time resolution. Polarization modulation IRLD
solves part of this problem by providing a 200 ms
time resolution and a much improved sensitivity, but at the expense of the
loss of the individual polarized spectra. This limits the structural
information available and can lead to quantification errors if
crystallization or conformational changes occur. We have very recently
demonstrated for the first time a novel method, polarization modulation
infrared structural absorbance spectroscopy (PM-IRSAS), that allows recording
the dichroic difference spectrum and the individual p- and s- polarized
spectra (and from these the structural absorbance spectrum) in a single 200 ms scan. PM-IRSAS therefore combines the speed and sensitivity
of PM-IRLD with the rich information of standard IRLD. We demonstrated its
performance by studying the deformation of poly(ethylene
terephthalate) (PET) thin films as well as its cold crystallization. The
importance of this work was recognized by the SAS Meggers
Award for the best paper published in Applied Spectroscopy in 2008.
Our current
efforts our directed at extending PM-IRSAS to new sampling modes besides
transmission. In addition to these instrumental developments, a current
application of PM-IRSAS is the study of the deformation mecha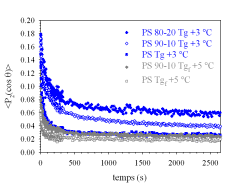 nisms and of the
broadening of the glass transition (Tg) in polymer blends. Blending polymers
offers a unique opportunity for optimizing their properties at low cost by
adjusting their composition and morphology. Blends usually display a
broadened Tg and the two components often present distinct dynamics in spite
of their miscibility. Two models attempt to explain these results: the
concentration fluctuation model, in which the blend contains nanometer-sized domains
enriched in one component and possessing a local Tg, and the Lodge-McLeish
model, in which self-concentration yields an effective Tg for each component.
In previous work, we have used PM-IRLD to study the deformation of blends of
PS with poly(vinyl methyl ether) (PVME), a system
with a very broad Tg. A master curve suggested that the end of the Tg zone (Tgf) is a better reference temperature than the
universally used Tg. We continue this work using PM-IRSAS by studying the
deformation of two other blends: PS with poly(phenylene oxide) (PPO) and poly(methyl methacrylate) with
poly(epichlorohydrin). These systems present a
narrow and a very broad Tg region, respectively, and thus constitute extreme
cases that will allow generalizing our interpretation to a vast class of
blends. nisms and of the
broadening of the glass transition (Tg) in polymer blends. Blending polymers
offers a unique opportunity for optimizing their properties at low cost by
adjusting their composition and morphology. Blends usually display a
broadened Tg and the two components often present distinct dynamics in spite
of their miscibility. Two models attempt to explain these results: the
concentration fluctuation model, in which the blend contains nanometer-sized domains
enriched in one component and possessing a local Tg, and the Lodge-McLeish
model, in which self-concentration yields an effective Tg for each component.
In previous work, we have used PM-IRLD to study the deformation of blends of
PS with poly(vinyl methyl ether) (PVME), a system
with a very broad Tg. A master curve suggested that the end of the Tg zone (Tgf) is a better reference temperature than the
universally used Tg. We continue this work using PM-IRSAS by studying the
deformation of two other blends: PS with poly(phenylene oxide) (PPO) and poly(methyl methacrylate) with
poly(epichlorohydrin). These systems present a
narrow and a very broad Tg region, respectively, and thus constitute extreme
cases that will allow generalizing our interpretation to a vast class of
blends.
Development and
application of PA-IR spectroscopy
For the last 30
years, infrared spectroscopy has been synonymous with FT-IR because of its
well-known advantages over dispersive instruments. It was recently recognized
that the newly available IR focal plane arrays (FPA) could be advantageously
coupled with dispersive IR instruments in a so-called planar array infrared
(PA-IR) spectrograph. PA-IR has two intrinsic advantages over FT-IR: 1) its
time resolution is almost 2 orders of magnitude better than that of
commercial FT-IRs; 2) it enables recording spectra from multiple beams or
sample locations at once. These advantages make PA-IR ideally suited for
studying irreversible phenomena at high speed, for high-throughput
experiments, and for recording 1D spatially resolved spectra.
We are one of the
three first groups worldwide (and the only one in Canada) working on the
development 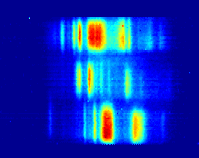 and application
of PA-IR spectroscopy. We have very recently shown that PA-IR provides more
than a tenfold time resolution improvement for polymer deformation studies as
compared to the fastest measurements ever published while still providing a
better signal-to-noise ratio. In this ongoing project, we follow the
structural evolution of polymers during their deformation using a
dual-polarization PA-IR spectrometer at deformation speeds much closer to
those relevant to industrial drawing processes. We also work on coupling
PA-IR with a dynamic mechanical analysis apparatus for performing
temperature-resolved PA-IR-DMA. Such experiments provide molecular-level
information about polymer viscoelasticity through the measurement of in-phase
(storage) and out-of-phase (loss) dynamic IR spectra. and application
of PA-IR spectroscopy. We have very recently shown that PA-IR provides more
than a tenfold time resolution improvement for polymer deformation studies as
compared to the fastest measurements ever published while still providing a
better signal-to-noise ratio. In this ongoing project, we follow the
structural evolution of polymers during their deformation using a
dual-polarization PA-IR spectrometer at deformation speeds much closer to
those relevant to industrial drawing processes. We also work on coupling
PA-IR with a dynamic mechanical analysis apparatus for performing
temperature-resolved PA-IR-DMA. Such experiments provide molecular-level
information about polymer viscoelasticity through the measurement of in-phase
(storage) and out-of-phase (loss) dynamic IR spectra.
Photoactive supramolecular
complexes
Bazuin et al. have recently shown that the
supramolecular complex between quaternized poly(vinyl pyridine) (PVP) and methyl orange (MO), a
photoactive azobenzene dye, forms a liquid crystal in spite of the absence of
a spacer between the main chain and mesogenic
group. Importantly, this material develops extremely high and stable
birefringence up to elevated temperatures when irradiated with polarized
laser radiation. We now use the newly developed PM-IRSAS technique to study
the orientation of both the MO and PVP under irradiation. We also explore the
possibilities of azobenzene supramolecular complexes in fibers and in new
photoactive materials.
Characterization and valorization
of the mussel byssus
The Canadian
mussel farming industry produces 22 000 tons of blue mussels worth
32.7M$ per year. These mussels attach to solid substrates, including the
farming lines, with assemblies of collagen-rich fibrous proteins called
byssus. These fibers possess high toughness and elasticity that vary with
environmental factors such as water temperature and turbulence. The molecular
origin of these property variations is still poorly understood. Byssus
fracture adversely affects the productivity of the farming industry with
losses up to 30%. In collaboration with biophysicist I. Marcotte (UQAM) and
biologist R. Tremblay (UQAR), we study the effect of environmental factors on
the byssus structure to better understanding the molecular origin of byssus
fracture. Furthermore, since byssuses constitute
~1% of the mussel weight and are currently treated as waste, we explore the
valorization of byssuses in new biomaterials.
Spectroscopic characterization of molecular glasses
While most polymers can readily
be prepared in the amorphous state, it is much less straightforward to compel
small molecules to form glasses. Strategies to prevent crystallization
include deep quenching from the melt state and fast
solvent evaporation, but the resulting samples tend to reverse to their
stable crystalline form with time, limiting their use in applications such as
drug excipients. Chemical strategies for preventing crystallization include
adding alkyl chains to limit intermolecular interactions, and lowering the
molecular symmetry to reduce packing efficiency. In collaboration with O.
Lebel (Royal Military College), we study glass-forming molecules based on a triazine core that contain several groups capable of
forming strong hydrogen bonds. We recently showed, using
temperature-controlled IR spectroscopy, that hydrogen bonding contributes to
frustrating the crystallization of 4,6-bis(3,5-dimethylphenylamino)-2-methoxy-1,3,5-triazine
through the formation of aggregates that pack poorly. It was observed that
disruption of the hydrogen bonds plays a key role in the physical changes
associated with the Tg of this molecular glass. We
are presently expanding this study to a series of twelve triazine
analogs from the melt state and fast
solvent evaporation, but the resulting samples tend to reverse to their
stable crystalline form with time, limiting their use in applications such as
drug excipients. Chemical strategies for preventing crystallization include
adding alkyl chains to limit intermolecular interactions, and lowering the
molecular symmetry to reduce packing efficiency. In collaboration with O.
Lebel (Royal Military College), we study glass-forming molecules based on a triazine core that contain several groups capable of
forming strong hydrogen bonds. We recently showed, using
temperature-controlled IR spectroscopy, that hydrogen bonding contributes to
frustrating the crystallization of 4,6-bis(3,5-dimethylphenylamino)-2-methoxy-1,3,5-triazine
through the formation of aggregates that pack poorly. It was observed that
disruption of the hydrogen bonds plays a key role in the physical changes
associated with the Tg of this molecular glass. We
are presently expanding this study to a series of twelve triazine
analogs
|