LEBEL GROUPResearch Interests |
Our research interests are directed toward catalysis in synthetic organic chemistry. The major aim of our research program is the development of new tools for the elaboration of structurally complex molecules that have useful pharmacological properties. We are also concerned by the development of new green processes involving the use of catalysts and the discovery of recyclable reagents. The use of organometallic complexes as catalysts to perform novel organic transformations that are not possible using more traditional reagents have expanded tremendously in the past 10 years. The use of as little as few milligrams of a catalyst to transform many grams of an organic substrates combined with the recycling of the catalyst, provide the development of an efficient green process. The development of multicatalytic processes that mimic the biosynthesis in living cells is another very exciting area. The fundamental aspect of the research is also instrumental, as the development of synthetic organic chemistry is directly related to the better understanding of reaction mechanism.
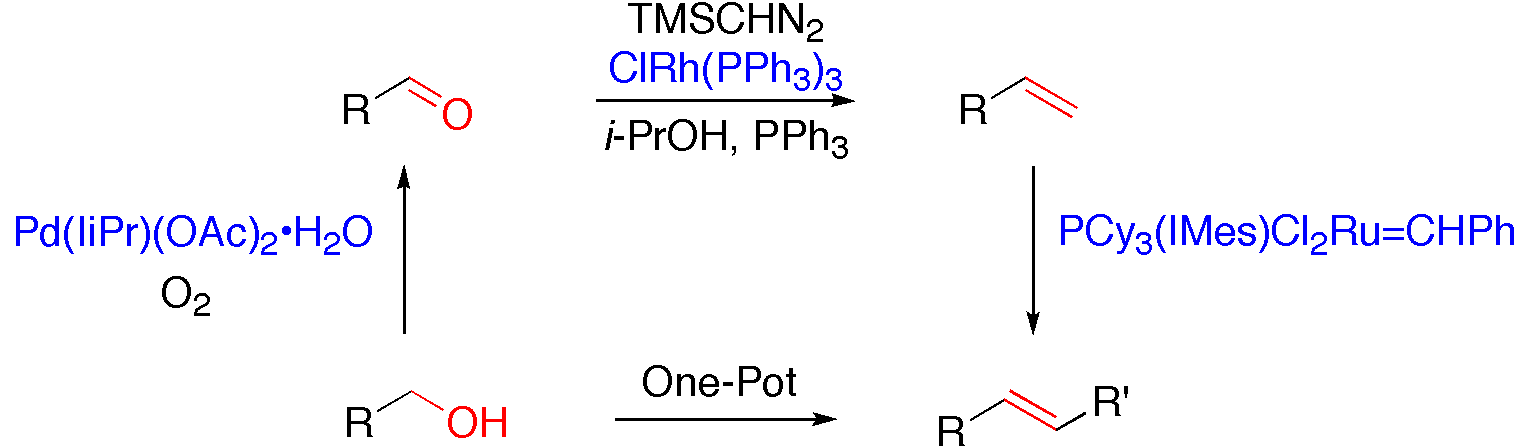
We are interested in developing new synthetic methodologies in organic chemistry based on transition metal catalyzed-processes. Alkene and nitrogen functionalities are particularly appealing to us, because of their preeminence in organic synthesis. For instance, we have disclosed a new synthetic strategy for the preparation of phosphorus ylide, that allow the methylenation of various carbonyl derivatives. Not only this method has a broad synthetic utility, but the mechanism of the methylenation reaction displays a unique mode of coordination between the diazo reagent and the rhodium catalyst. We are also interested at developing new multicatalytic procedures, a conceptually new synthetic process that is widely found in nature. Indeed, we developed a series of one-pot procedures that combined various transition metal catalysts to achieve specific reactions. It provided the first catalytic synthetic procedure for the direct conversion of alcohols into alkenes and highlights the compatibility of various transition metal complexes. The importance and the power of such procedures were also highlighted since, in most cases, the yield of the one-pot process is higher that the combined yield of the step-by-step approach.
We are also involved in the more general area of the coordination and reaction between nitrogen atoms and transition metal complexes, which are well characterized in inorganic chemistry, but have never been looked at from the point of view of organic reactions. Our goal is to pursue the investigation of the activation of nitrogen derivatives (such as diazo or azide compounds) with transition metal complexes, to develop new catalysts for 1,2-rearrangements. The common driving force of the Wolff, Curtius and Schmidt rearrangements is the loss of nitrogen from either a diazocarbonyl or an acyl azide compound. These rearrangements have been extensively used in organic chemistry to synthesize various nitrogen products. Our goal is to study the effect of transition metal catalysts on these processes, with the idea that coordination with nitrogen could accelerate the reaction.
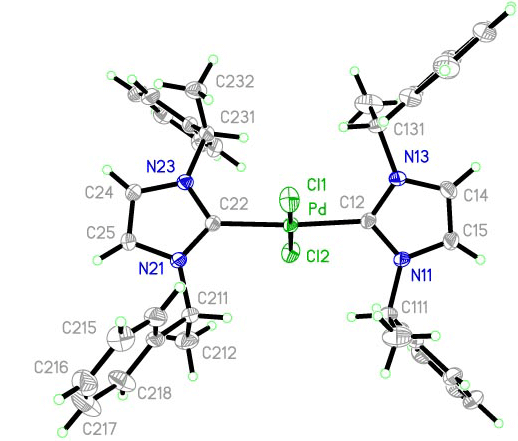
Another aspect of our research is the design of new chiral ligands for transition metals in order to develop efficient chiral catalysts for the stereocontrol of organic reactions. Our research provides a strong exposure to organic chemistry as well as organometallic chemistry and to a broad range of sophisticated techniques, including high filed NMR spectroscopy, mass spectrometry and X-ray crystallography.